30-8-2023 (LONDON) In a groundbreaking development, the United Kingdom’s state-run National Health Service (NHS) is poised to revolutionize cancer treatment worldwide. It will become the global pioneer in administering an innovative cancer-fighting injection that promises to drastically reduce treatment durations, potentially by up to 75%.
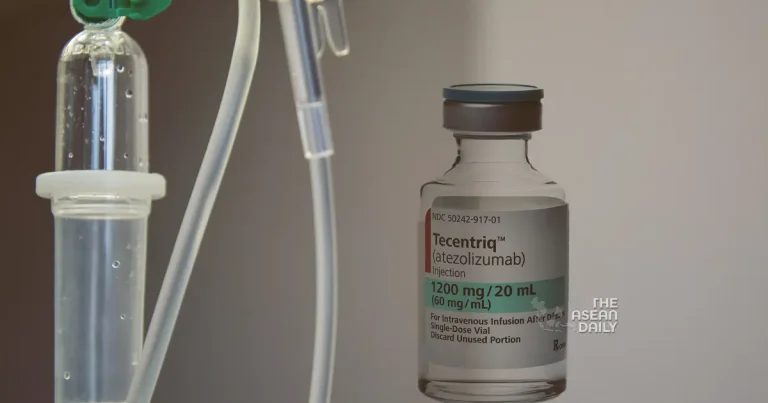
The momentous decision comes in the wake of an official green light from the Medicines and Healthcare Products Regulatory Agency (MHRA). NHS England, in an announcement made on Tuesday, revealed that this revolutionary approach would be offered to hundreds of eligible cancer patients across England. The treatment in question involves the use of atezolizumab, an immunotherapy drug, and will be administered as a “subcutaneous” injection, delivering not only enhanced convenience but also liberating precious time for cancer care teams.
Dr. Alexander Martin, a consultant oncologist at the West Suffolk NHS Foundation Trust, expressed his enthusiasm about the milestone: “This approval will not only allow us to deliver convenient and faster care for our patients but will enable our teams to treat more patients throughout the day.”
Traditionally, atezolizumab, also known by its trade name Tecentriq, has been administered intravenously, directly into patients’ veins through a drip. This method often consumed approximately 30 to 60 minutes, sometimes posing challenges in locating a suitable vein for certain patients.
Dr. Marius Scholtz, Medical Director at Roche Products, the company behind atezolizumab, highlighted the significant time-saving aspect of the new injection method: “It takes approximately seven minutes, compared with 30 to 60 minutes for the current method of an intravenous infusion.”
Atezolizumab, developed by Genentech, a subsidiary of Roche, is an immunotherapy drug designed to bolster a patient’s immune system to actively seek out and destroy cancerous cells. Currently, the treatment is offered via transfusion to NHS patients grappling with various cancer types, including lung, breast, liver, and bladder cancers.
NHS England anticipates that the majority of the approximately 3,600 patients who initiate atezolizumab treatment each year in England will opt for the time-saving injection. However, it’s important to note that patients receiving intravenous chemotherapy in conjunction with atezolizumab may continue with the transfusion method.