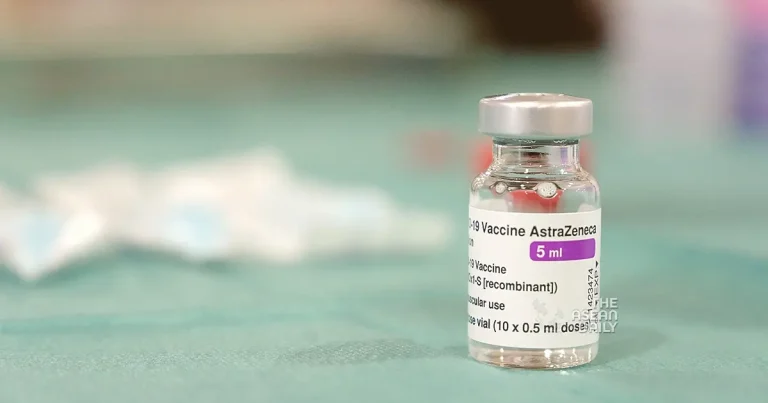
8-5-2024 (LONDON) AstraZeneca, the Anglo-Swedish pharmaceutical giant, announced on Tuesday (May 7) that it has initiated the worldwide withdrawal of its COVID-19 vaccine, Vaxzevria, citing a “surplus of available updated vaccines” since the pandemic.
The company stated that due to the availability of multiple, variant-specific COVID-19 vaccines developed since the initial rollout, there is now a surplus of updated vaccines, leading to a decline in demand for Vaxzevria. Consequently, the vaccine is no longer being manufactured or supplied.
AstraZeneca revealed that it would proceed to withdraw the marketing authorizations for Vaxzevria within Europe, marking a notable shift in the company’s strategy regarding its COVID-19 vaccine.
According to media reports, the pharmaceutical firm had previously acknowledged in court documents that the vaccine could cause side effects such as blood clots and low blood platelet counts, although these occurrences were described as “very rare.”
The Telegraph, a British newspaper, reported that AstraZeneca’s application to withdraw the vaccine was made on March 5, and the withdrawal came into effect on May 7. The legal document, submitted in February as part of a class-action lawsuit, stated that the company’s vaccine “can, in very rare cases, cause TTS” – a condition known as thrombotic thrombocytopenia syndrome, which the World Health Organization (WHO) has classified as a “serious and life-threatening adverse event.”
It is worth noting that Singapore has not authorized the use of AstraZeneca’s COVID-19 vaccine. In April 2023, Health Minister On Ye Kung addressed this matter in a parliamentary reply, citing concerns about thrombosis as the reason for not approving the vaccine’s use in the country.
As AstraZeneca moves away from its COVID-19 vaccine, the company has been shifting its focus to other areas, including respiratory syncytial virus vaccines and obesity drugs. Last year, the London-listed firm made several deals to expand its presence in these fields, reflecting a strategic reorientation in the face of declining growth in COVID-19 medicine sales.